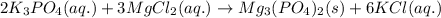Chemistry, 19.12.2019 20:31 raylynnreece5052
Aqueous potassium phosphate was mixed with aqueous magnesium chloride, and a crystallized magnesium phosphate product was formed. consider the other product and its phase, and then write the balanced molecular equation for this precipitation reaction. express your answer as a chemical equation including phases.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
You know the right answer?
Aqueous potassium phosphate was mixed with aqueous magnesium chloride, and a crystallized magnesium...
Questions in other subjects:


Chemistry, 20.04.2021 19:00

Mathematics, 20.04.2021 19:00

Mathematics, 20.04.2021 19:00



Chemistry, 20.04.2021 19:00

Mathematics, 20.04.2021 19:00