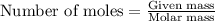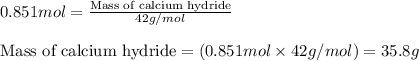Chemistry, 19.12.2019 20:31 cameronrandom00
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g)how many grams of cah2 are needed to generate 48.0 l of h2 gas at a pressure of 0.888 atm and a temperature of 32°c? calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g)how many grams of cah2 are needed to generate 48.0 l of h2 gas at a pressure of 0.888 atm and a temperature of 32°c? 35.850.70.85171.7143

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 23:30, billybob8514
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2...
Questions in other subjects:




Mathematics, 22.04.2020 20:22


Physics, 22.04.2020 20:22

Biology, 22.04.2020 20:22

Biology, 22.04.2020 20:22

Mathematics, 22.04.2020 20:23

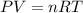
![32^oC=[32+273]K=305K](/tpl/images/0426/4065/40af0.png)
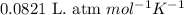

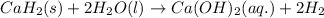
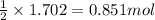 of calcium hydride
of calcium hydride