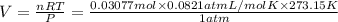Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Zinc will react with hydrochloric acid to produce hydrogen gas. zn(s)+2hcl(aq)⟶zncl2(aq)+h2(g)what i...
Questions in other subjects:







Mathematics, 07.06.2021 22:30



History, 07.06.2021 22:30

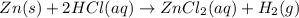
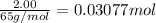
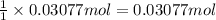 of hydrogen gas
of hydrogen gas