Chemistry, 19.12.2019 05:31 colochaortiz20p7cajw
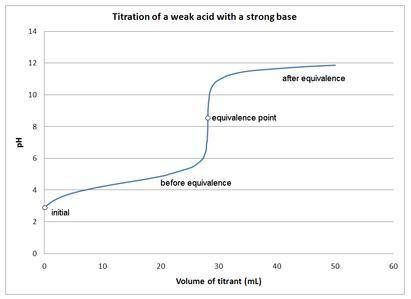
Aweak acid only is present: a. before the equivalence point of the titration of a strong acid with strong base. b. at the equivalence point of the titration of a weak base with strong acid. c. after the equivalence point of a strong acid – strong base titration. d. after the equivalence point of a weak acid – strong base titration.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3


Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 19:20, johnkings140
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
Aweak acid only is present: a. before the equivalence point of the titration of a strong acid with...
Questions in other subjects:


Mathematics, 06.03.2021 07:40


Mathematics, 06.03.2021 07:40


Arts, 06.03.2021 07:40

Mathematics, 06.03.2021 07:40

Mathematics, 06.03.2021 07:40

Mathematics, 06.03.2021 07:40