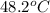Chemistry, 19.12.2019 02:31 mlarsen5000
In a coffee-cup calorimeter, 150.0 ml of 0.50 m hcl is added to 50.0 ml of 1.00 m naoh to make 200.0 g solution at an initial temperature of 48.2°c. if the enthalpy of neutralization for the reaction between a strong acid and a strong base is −56 kj/mol, calculate the final temperature of the calorimeter contents. assume the specific heat capacity of the solution is 4.184 j°c⁻¹ g⁻¹ and assume no heat loss to the surroundings.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
In a coffee-cup calorimeter, 150.0 ml of 0.50 m hcl is added to 50.0 ml of 1.00 m naoh to make 200.0...
Questions in other subjects:

English, 08.12.2020 18:10

Biology, 08.12.2020 18:10


Mathematics, 08.12.2020 18:10





Mathematics, 08.12.2020 18:10

Mathematics, 08.12.2020 18:10


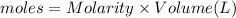
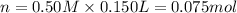





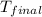 = final temperature =
= final temperature = 
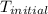 = initial temperature =
= initial temperature =