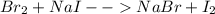Unknown halogen x2 was added to each of two test tubes which contained aqueous nacl and aqueous nai, respectively. when hexane was added to the test tubes, an orange color developed in the top layer of the first test tube, while purple developed in the second.
a. identify x2.
b. write a balanced equation for each reaction which occurred.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
Unknown halogen x2 was added to each of two test tubes which contained aqueous nacl and aqueous nai,...
Questions in other subjects:




Computers and Technology, 18.04.2020 16:58

English, 18.04.2020 16:58

Engineering, 18.04.2020 16:58

Mathematics, 18.04.2020 16:58

English, 18.04.2020 16:58

Biology, 18.04.2020 16:58

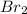
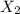 is
is 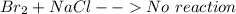 (because bromine is less reactive than chlorine)
(because bromine is less reactive than chlorine)