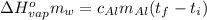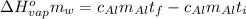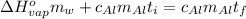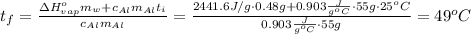Chemistry, 18.12.2019 06:31 hiiliohi3823
Suppose that 0.48 g of water at 25 ∘ c condenses on the surface of a 55- g block of aluminum that is initially at 25 ∘ c . if the heat released during condensation goes only toward heating the metal, what is the final temperature (in degrees celsius) of the metal block? (the specific heat capacity of aluminum, c s, al , is 0.903 j/(g⋅ ∘ c) .) express the temperature in degrees celsius to two significant figures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1


Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
Suppose that 0.48 g of water at 25 ∘ c condenses on the surface of a 55- g block of aluminum that is...
Questions in other subjects:



Chemistry, 08.04.2021 17:50


History, 08.04.2021 17:50


Health, 08.04.2021 17:50



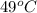
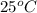 , the heat of vaporization of water is given by:
, the heat of vaporization of water is given by: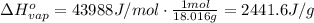
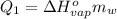
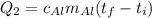
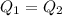 or:
or: