
Chemistry, 18.12.2019 05:31 daniella999sandoval
Ch3ch2ch2cl exhibits hydrogen bonding\rm ch_3ch_2ch_2cl exhibits blank, which are stronger than blank, so it has a higher boiling point than \rm ch_4 and \rm ch_3ch_2ch_3., which are stronger than dipole-dipole forces\rm ch_3ch_2ch_2cl exhibits blank, which are stronger than blank, so it has a higher boiling point than \rm ch_4 and \rm ch_3ch_2ch_3., so it has a higher boiling point than ch4 and ch3ch2ch3.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
Ch3ch2ch2cl exhibits hydrogen bonding\rm ch_3ch_2ch_2cl exhibits blank, which are stronger than blan...
Questions in other subjects:

Spanish, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40

Spanish, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40

English, 23.02.2021 02:40


History, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40


Mathematics, 23.02.2021 02:40

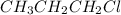 , 1-chloropropane, exhibits mainly dipole-dipole forces, chlorine is too big to be able to form hydrogen bonds. Dipole-dipole forces occur in this compound due to the fact that chlorine is strongly electronegative and polarizing, so the two molecules would form dipoles. Dipole-dipole forces are stronger than London dispersion forces, therefore, 1-chloropropane has a higher boiling point than methane or propane.
, 1-chloropropane, exhibits mainly dipole-dipole forces, chlorine is too big to be able to form hydrogen bonds. Dipole-dipole forces occur in this compound due to the fact that chlorine is strongly electronegative and polarizing, so the two molecules would form dipoles. Dipole-dipole forces are stronger than London dispersion forces, therefore, 1-chloropropane has a higher boiling point than methane or propane.

