
Chemistry, 18.12.2019 04:31 broomssymphonie
There are three ways in which to define acids and bases: the arrhenius concept, the brønsted-lowry concept, and the lewis concept. arrhenius acids are substances that, when dissolved in water, increase the concentration of the h+ ion; arrhenius bases are substances that, when dissolved in water, increase the concentration of the oh− ion. brønsted-lowry acids are substances that can donate a proton (h+) to another substance; brønsted-lowry bases are substances that can accept a proton (h+). a lewis acid is an electron-pair acceptor, and a lewis base is an electron-pair donor. part a using the arrhenius concept of acids and bases, identify the arrhenius acid and base in each of the following reactions: 2koh(aq)+h2so4(aq)→k2so4(aq)+2h2o(l ) nh3(g)+hcl(g)→nh4cl(s)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


You know the right answer?
There are three ways in which to define acids and bases: the arrhenius concept, the brønsted-lowry...
Questions in other subjects:


Mathematics, 24.07.2019 09:50



English, 24.07.2019 09:50

Mathematics, 24.07.2019 09:50




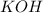 acts as base.
acts as base.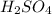 acts as acid.
acts as acid.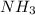 acts as base.
acts as base.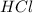 acts as acid.
acts as acid.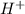 .
.
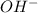 .
.
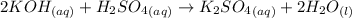
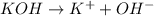 and hence, acts as base.
and hence, acts as base.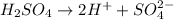 and hence, acts as acid.
and hence, acts as acid.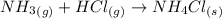
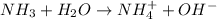 and hence, acts as base.
and hence, acts as base.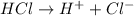 and hence, acts as acid.
and hence, acts as acid.

