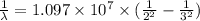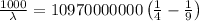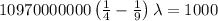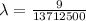Chemistry, 18.12.2019 01:31 tylermdons
The energy of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -ry/n^2
in this equation, ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron.
a) calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=2 to an orbital with n=3 . round your answer to significant digits.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:31, CassidgTab
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3

Chemistry, 23.06.2019 10:20, kyliemorgan8623
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
The energy of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -r...
e= -r...
Questions in other subjects:


Mathematics, 10.11.2020 16:20






Social Studies, 10.11.2020 16:20


Biology, 10.11.2020 16:20

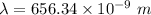
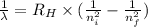
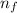 = 3
= 3
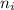 = 2
= 2