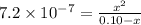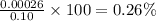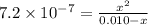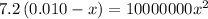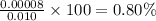Percent ionization
percent ionization for a weak acid (ha) is determined by the following form...

Chemistry, 18.12.2019 01:31 mariamalakozay603
Percent ionization
percent ionization for a weak acid (ha) is determined by the following formula:
percent ionization=[ha] ionized[ha] initial×100%
for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.
a certain weak acid, ha, has a ka value of 7.6×10−7.
part a
calculate the percent ionization of ha in a 0.10 m solution.
express your answer as a percent using two significant figures.
%
submithintsmy answersgive upreview part
part b
calculate the percent ionization of ha in a 0.010 m solution.
express your answer as a percent using two significant figures.
%

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 08.11.2019 01:31

History, 08.11.2019 01:31


Mathematics, 08.11.2019 01:31

History, 08.11.2019 01:31


Mathematics, 08.11.2019 01:31

Mathematics, 08.11.2019 01:31

Mathematics, 08.11.2019 01:31



![K_{a}=\frac {\left [ H^{+} \right ]\left [ {A}^- \right ]}{[HA]}](/tpl/images/0423/3691/59869.png)