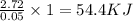Chemistry, 18.12.2019 01:31 mallorynichole19
When a student mixes 50 ml of 1.0 m hcl and 50 ml of 1.0 m naoh in a coffee-cup calorimeter, the temperature of the resultant solution increases from 21.0 °c to 27.5 °c. calculate the enthalpy change for the reaction in kj per mol of hcl, assuming that the calorimeter loses only a negligible quantity of heat. the total volume of the solution is 100 ml, its density is 1.0 g/ml, and its specific heat is 4.18 j/g*k.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 23.06.2019 09:00, Drellibus70011
Agust of wind blowing east pushes against a ball. when will the wind do work on the ball? when the ball moves to the east when the ball moves to the north when the ball stays in one place when the ball moves north or south
Answers: 1
You know the right answer?
When a student mixes 50 ml of 1.0 m hcl and 50 ml of 1.0 m naoh in a coffee-cup calorimeter, the tem...
Questions in other subjects:



Mathematics, 27.05.2021 23:50

History, 27.05.2021 23:50


English, 27.05.2021 23:50

French, 27.05.2021 23:50



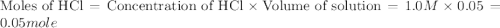
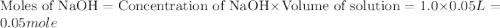

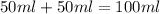
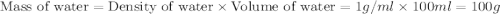
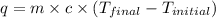
 = specific heat of water =
= specific heat of water = 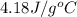
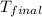 = final temperature of water =
= final temperature of water = 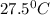
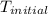 = initial temperature of metal =
= initial temperature of metal = 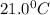
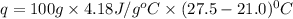
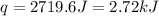
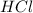 :
: