
Chemistry, 17.12.2019 23:31 cathydaves
Carbon monoxide reacts with oxygen gas to form carbon dioxide according to the following chemical equation. 2co(g)+o2(g)⟶2co2(g) how many ml of oxygen gas will remain in the reaction vessel when 82.4ml of carbon monoxide gas reacts with 80.0ml of oxygen gas to form carbon dioxide under constant pressure and temperature?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

You know the right answer?
Carbon monoxide reacts with oxygen gas to form carbon dioxide according to the following chemical eq...
Questions in other subjects:

Physics, 03.09.2021 20:20

Mathematics, 03.09.2021 20:20


English, 03.09.2021 20:20

Mathematics, 03.09.2021 20:20

Mathematics, 03.09.2021 20:20



Mathematics, 03.09.2021 20:20

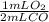 = 41.2 mL O₂
= 41.2 mL O₂


