
Chemistry, 17.12.2019 22:31 missy922527
Consider the following system at equilibrium where h° = -87.9 kj, and kc = 83.3, at 500 k. pcl3(g) + cl2(g) pcl5(g) when 0.17 moles of cl2(g) are added to the equilibrium system at constant temperature: the value of kc the value of qc kc. the reaction must run in the forward direction to restablish equilibrium. run in the reverse direction to restablish equilibrium. remain the same. it is already at equilibrium. the concentration of pcl3 will

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Consider the following system at equilibrium where h° = -87.9 kj, and kc = 83.3, at 500 k. pcl3(g) +...
Questions in other subjects:



History, 05.05.2020 16:04





History, 05.05.2020 16:04


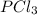 will decrease.
will decrease.


