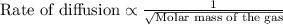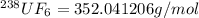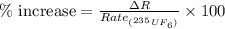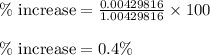Chemistry, 17.12.2019 21:31 anaislewis8296
During the discussion of gaseous diffusion for enriching uranium, it was claimed that 235uf6 diffuses 0.4% faster than 238uf6. show the calculation that supports this value. the molar mass of 235uf6 = 235.043930 + 6 x 18.998403 = 349.034348 g/mol, and the molar mass of 238uf6 = 238.050788 + 6 x 18.998403 = 352.041206 g/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
During the discussion of gaseous diffusion for enriching uranium, it was claimed that 235uf6 diffuse...
Questions in other subjects:

Geography, 29.09.2020 03:01


Mathematics, 29.09.2020 03:01


Mathematics, 29.09.2020 03:01

Mathematics, 29.09.2020 03:01

Physics, 29.09.2020 03:01


Biology, 29.09.2020 03:01

Mathematics, 29.09.2020 03:01

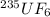 is 0.4 % faster than the rate of diffusion of
is 0.4 % faster than the rate of diffusion of