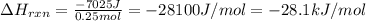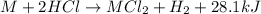Chemistry, 17.12.2019 21:31 colestout2993
Consider the reaction: m + 2hcl → mcl2 + h2
when 0.25 mol of the metal, m, reacted with an aqueous hcl solution (the hcl is in excess), the temperature of the solution rose because the reaction produced 7025 j of heat. what is ∆h in kj per mol of m for this reaction? (hint: is this reaction exothermic or endothermic? )

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2



Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Consider the reaction: m + 2hcl → mcl2 + h2
when 0.25 mol of the metal, m, reacted wit...
when 0.25 mol of the metal, m, reacted wit...
Questions in other subjects:

Mathematics, 21.08.2020 22:01

English, 21.08.2020 22:01


Mathematics, 21.08.2020 22:01



Mathematics, 21.08.2020 22:01

Mathematics, 21.08.2020 22:01

Mathematics, 21.08.2020 22:01

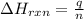
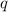 = amount of heat released = -7025 J
= amount of heat released = -7025 J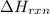 = enthalpy change of the reaction
= enthalpy change of the reaction