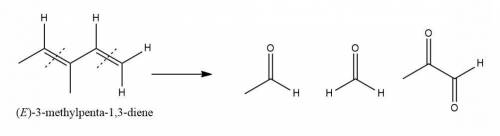
An unknown compound with empirical formula c3h5 was treated with br2/ccl4. the bromine solution went from orangish/red to clear immediately at room temperature. upon treatment with o3 followed by work-up with dimethylsulfide the following products were identified. from the information provided what is/are the most likely structure(s) for this unknown compound.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 02:50, giavanleer14
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
An unknown compound with empirical formula c3h5 was treated with br2/ccl4. the bromine solution went...
Questions in other subjects:

Mathematics, 19.08.2019 21:50

Mathematics, 19.08.2019 21:50



Physics, 19.08.2019 21:50

Chemistry, 19.08.2019 21:50

Mathematics, 19.08.2019 21:50

English, 19.08.2019 21:50


 .
.