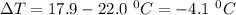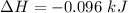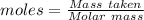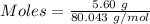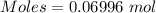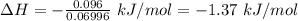Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 is dissolved in 100g of water at 22.0c; the temperature falls to 17.9c. assuming that the specific heat capacity of the solution is 4.18 j/(g*k), calculate the enthalpy of dissolution of nh4no3, in kj/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, annsmith66
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:50, agm0102
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 i...
Questions in other subjects:


Mathematics, 20.04.2020 22:32

Mathematics, 20.04.2020 22:32


Mathematics, 20.04.2020 22:32

Mathematics, 20.04.2020 22:32

Spanish, 20.04.2020 22:32


Mathematics, 20.04.2020 22:32

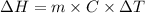
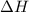 is the enthalpy of dissolution of [tex[NH_4NO_3[/tex]
is the enthalpy of dissolution of [tex[NH_4NO_3[/tex] is the temperature change
is the temperature change