
The reaction between c6h12o6 and o2 is represented by the balanced equation above. in an experiment, 0.30mol of co2 was produced from the reaction of 0.05mol of c6h12o6 with excess o2. the reaction was repeated at the same temperature and in the same container, but this time 0.60mol of co2 was produced. which of the following must be true

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1


Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
The reaction between c6h12o6 and o2 is represented by the balanced equation above. in an experiment,...
Questions in other subjects:


Mathematics, 12.08.2020 07:01


Mathematics, 12.08.2020 07:01



Computers and Technology, 12.08.2020 07:01


Mathematics, 12.08.2020 07:01

Biology, 12.08.2020 07:01

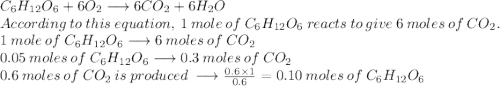
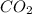 is produced when 0.1 mol of
is produced when 0.1 mol of 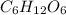 reacts with excess oxygen.
reacts with excess oxygen.

