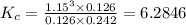Chemistry, 17.12.2019 05:31 Shadow0202
Hydrogen is prepared commercially by the reaction of methane and water vapor at elevated temperatures. ch₄ + h₂ ⇌ 3h₂ + what is the equilibrium constant for the reaction if a mixture at equilibrium contains gases with the following concentrations: ch₄, 0.126 m; h₂o, 0.242 m; co, 0.126 m; h₂ 1.15 m, at a temperature of 760 °c?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 06:00, tytianadyson74
What volume of argon gas is equal to 1.60 grams of argon
Answers: 1
You know the right answer?
Hydrogen is prepared commercially by the reaction of methane and water vapor at elevated temperature...
Questions in other subjects:



Mathematics, 20.07.2019 13:00



Mathematics, 20.07.2019 13:00


English, 20.07.2019 13:00


![[CH_4]=0.126\ M](/tpl/images/0421/9784/de111.png)
![[H_2O]= 0.242\ M](/tpl/images/0421/9784/d80cb.png)
![[CO]= 0.126\ M](/tpl/images/0421/9784/495e0.png)
![[H_2]= 1.15\ M](/tpl/images/0421/9784/0632f.png)
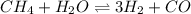
![K_{c}=\frac {\left [ H_2 \right ]^3\left [ CO \right ]}{\left [ CH_4 \right ]\left [ H_2O \right ]}](/tpl/images/0421/9784/6aeab.png)