Chemistry, 17.12.2019 04:31 princessx7543
Given that  [br(g)] = 111.9 kj ⋅ mol⁻¹
[br(g)] = 111.9 kj ⋅ mol⁻¹ [br(g)] = 111.9 kj⋅mol⁻¹
[br(g)] = 111.9 kj⋅mol⁻¹ [c(g)] = 716.7 kj ⋅ mol⁻¹
[c(g)] = 716.7 kj ⋅ mol⁻¹ [c(g)] = 716.7 kj⋅mol⁻¹
[c(g)] = 716.7 kj⋅mol⁻¹ [cbr₄(g)] = 29.4 kj ⋅ mol⁻¹
[cbr₄(g)] = 29.4 kj ⋅ mol⁻¹ [cbr₄(g)] = 29.4 kj⋅mol⁻¹ calculate the average molar bond enthalpy of the carbon–bromine bond in a cbr₄ molecule.
[cbr₄(g)] = 29.4 kj⋅mol⁻¹ calculate the average molar bond enthalpy of the carbon–bromine bond in a cbr₄ molecule.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 02:00, hayleebeals50
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

You know the right answer?
Given that [tex]\delta h^o_f[/tex] [br(g)] = 111.9 kj ⋅ mol⁻¹[tex]\delta h^o_f[/tex] [br(g)] = 111.9...
Questions in other subjects:

Health, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

History, 14.09.2020 22:01

Social Studies, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

History, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

History, 14.09.2020 22:01

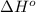
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0421/8523/45485.png)
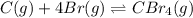
![\Delta H^o_{rxn}=[(n_{(CBr_4)}\times \Delta H^o_f_{(CBr_4)})]-[(n_{(Br)}\times \Delta H^o_f_{(Br)})+(n_{(C)}\times \Delta H^o_f_{(C)})]](/tpl/images/0421/8523/304b7.png)
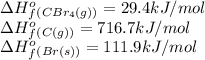
![\Delta H^o_{rxn}=[(1\times 29.4)]-[(4\times 111.9)+(1\times 716.7)]=-1134.9kJ/mol](/tpl/images/0421/8523/33ec8.png)
![\Delta H=-[4\times B.E_{C-Br}]](/tpl/images/0421/8523/b980a.png)
![-1134.9=-[4\times B.E_{C-Br}]](/tpl/images/0421/8523/74d8f.png)