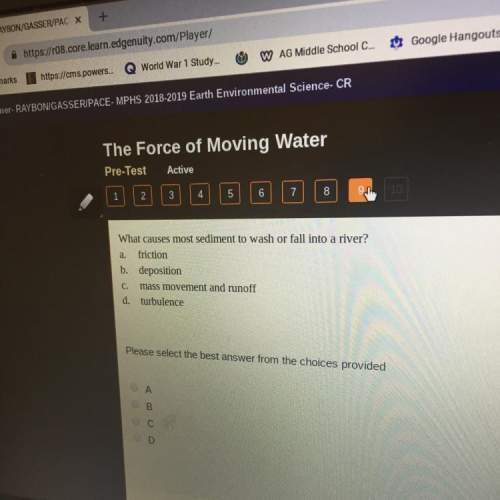
Nthe first step of the industrial process for making nitric acid, ammonia reacts with oxygen in the presence of a suitable catalyst to form nitric oxide and water vapor: 4 nh31g2+5 o21g2¡4 no1g2+6 h2o1g2how many liters of nh31g2 at 850 °c and 5.00 atm are required to react with 1.00 mol of o21g2 in this reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Nthe first step of the industrial process for making nitric acid, ammonia reacts with oxygen in the...
Questions in other subjects:

Mathematics, 04.07.2019 14:50

English, 04.07.2019 14:50

Computers and Technology, 04.07.2019 14:50


Chemistry, 04.07.2019 14:50


Social Studies, 04.07.2019 14:50

Chemistry, 04.07.2019 14:50


 = 1.00 moles
= 1.00 moles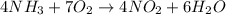
 moles of ammonia
moles of ammonia 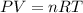
 required.
required.