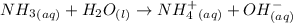Chemistry, 17.12.2019 04:31 brionnashelp
An aqueous solution of ammonia is found to be basic. this observation can be explained by the net ionic equationhno3(aq) + h2o(l) → no3-(aq) + h3o+(aq).nh4+(aq) + h2o(l) → nh3(aq) + h3o+(aq).no3-(aq) + h2o(l) → hno3(aq) + oh-(aq).nh3(aq) + h2o(l) → nh4+(aq) + oh-(aq).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:40, ricardoamora54
Who is better, messi or cristiano, i need this for a chemistry class. asap
Answers: 1


You know the right answer?
An aqueous solution of ammonia is found to be basic. this observation can be explained by the net io...
Questions in other subjects:

English, 17.01.2020 23:31


Physics, 17.01.2020 23:31

Mathematics, 17.01.2020 23:31


Mathematics, 17.01.2020 23:31



Mathematics, 17.01.2020 23:31

History, 17.01.2020 23:31