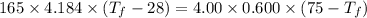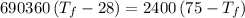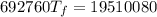Chemistry, 17.12.2019 04:31 aaronjcerrato
A4.00 g sample of a metal (specific heat = 0.600 j g-1°c-1 is heated to 75 degrees celcius and then dropped into 165 g of water in a calorimeter. what is the final temperature of the water if the initial temperature is 28 degrees celcius? the specific heat capacity of water is 4.184 j/g.°c.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, IsabellaGracie
True or false, the three major scales used to measure earthquakes are mercalli scale, richter scale and magnitude scale
Answers: 2

Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
A4.00 g sample of a metal (specific heat = 0.600 j g-1°c-1 is heated to 75 degrees celcius and then...
Questions in other subjects:

Mathematics, 27.08.2019 08:00


History, 27.08.2019 08:00


Mathematics, 27.08.2019 08:00

Social Studies, 27.08.2019 08:00

History, 27.08.2019 08:00


Mathematics, 27.08.2019 08:00

Geography, 27.08.2019 08:00