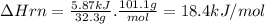Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g of kno3 is dissolved in 243 g of water at 23.00 °c. kno3(s)+h2o(aq) > koh(aq)+hno3(aq)the temperature of the resulting solution decreases to 17.90 °c. assume the resulting solution has the same specific heat as water, 4.184 j/(g·°c), and that there is negligible heat loss to the surroundings.1. how much heat was released by the solution? 2. what is the enthalpy of the reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2


Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1
You know the right answer?
Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g...
Questions in other subjects: