

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
You know the right answer?
Express the equilibrium constant for the following reaction.2 k(s) + 2 h2o(l) ↔ 2 koh(aq) + h2(g)k =...
Questions in other subjects:


Social Studies, 21.01.2021 20:40

Mathematics, 21.01.2021 20:40




Mathematics, 21.01.2021 20:40



History, 21.01.2021 20:40

![K=[KOH]^2[H_2]](/tpl/images/0421/6907/b9417.png)
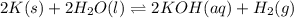
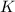 will be,
will be,

