Chemistry, 17.12.2019 01:31 gchippewa81
Find the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h8(g) + 5o2(g) → 3co2(g) + 4h2o(g) ∆h = −2043 kj c(s) + o2(g) → co2(g) ∆h = −393.5 kj 2h2(g) + o2(g) → 2h2o(g) ∆h = −483.6 kj express your answer in kj.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 23.06.2019 06:00, fjsdfj1284
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
Find the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h8...
Questions in other subjects:


Mathematics, 19.11.2020 08:20


Mathematics, 19.11.2020 08:20

Mathematics, 19.11.2020 08:20





 for the reaction is -104.7 kJ.
for the reaction is -104.7 kJ.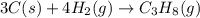 ;
; 
 ;
; 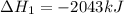
 ;
; 
 ;
; 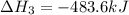
 ;
;