
Consider the following reaction at equilibrium. what effect will increasing the temperature have on the system? fe3o4(s) + co(g) ↔ 3 feo(s) + co2(g) δh°= +35.9 kjthe equilibrium constant will decrease. no effect will be observed. the reaction will shift to the right in the direction of products. the equilibrium constant will increase. the reaction will shift to the left in the direction of reactants

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Consider the following reaction at equilibrium. what effect will increasing the temperature have on...
Questions in other subjects:










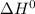 of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.
of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.

