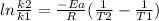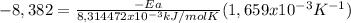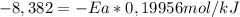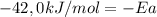Chemistry, 16.12.2019 21:31 krandall232
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction.23.8 kj/mol11.5 kj/mol12.5 kj/mol42.0 kj/mol58.2 kj/mol

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate con...
Questions in other subjects:


History, 13.04.2021 19:10


History, 13.04.2021 19:10

Mathematics, 13.04.2021 19:10

Mathematics, 13.04.2021 19:10

Mathematics, 13.04.2021 19:10