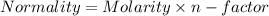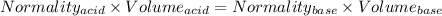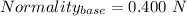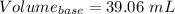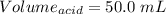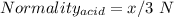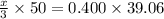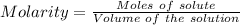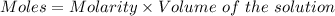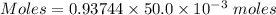Chemistry, 16.12.2019 19:31 secondcolinmills005
You are given a 1.00 g sample of an unknown tri-protic acid, which you dissolve in 50.0 ml of water containing phenolphthalein indicator. you titrate the acid solution with standardized 0.400 m koh(aq). it requires 39.06 ml of the koh solution to produce a light pink indicator color. what is the molecular weight of the unknown acid?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:50, limelight11
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1


You know the right answer?
You are given a 1.00 g sample of an unknown tri-protic acid, which you dissolve in 50.0 ml of water...
Questions in other subjects:

English, 24.11.2020 05:40



History, 24.11.2020 05:40


English, 24.11.2020 05:40

Mathematics, 24.11.2020 05:40


Mathematics, 24.11.2020 05:40

Physics, 24.11.2020 05:40