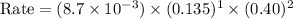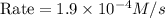Chemistry, 16.12.2019 19:31 pearpeaerrr1993
Consider the following reaction between mercury(ii) chloride and oxalate ion: 2hgcl2(aq)+c2o2−4(aq)→2cl−(aq)+2co2 (g)+hg2cl2(s) the initial rate of this reaction was determined for several concentrations of hgcl2 and c2o2−4, and the following rate data were obtained for the rate of disappearance of c2o2−4:

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

You know the right answer?
Consider the following reaction between mercury(ii) chloride and oxalate ion: 2hgcl2(aq)+c2o2−4(aq)...
Questions in other subjects:



Geography, 28.08.2020 14:01


Mathematics, 28.08.2020 14:01

SAT, 28.08.2020 14:01


English, 28.08.2020 14:01

Mathematics, 28.08.2020 14:01

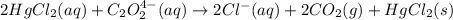
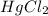 and
and 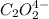 , and the following rate data were obtained for the rate of disappearance of
, and the following rate data were obtained for the rate of disappearance of 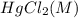
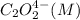 Rate (M/s)
Rate (M/s)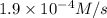
![\text{Rate}=k[HgCl_2]^a[C_2O_2^{4-}]^b](/tpl/images/0420/9839/2a902.png)
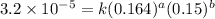 ....(1)
....(1)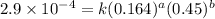 ....(2)
....(2)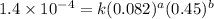 ....(3)
....(3)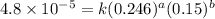 ....(4)
....(4)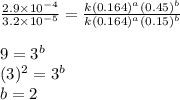
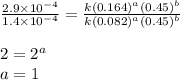
![\text{Rate}=k[HgCl_2]^1[C_2O_2^{4-}]^2](/tpl/images/0420/9839/2292e.png)
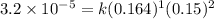
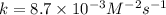
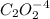 is 0.40 M.
is 0.40 M.