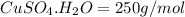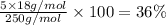Chemistry, 16.12.2019 19:31 jazprincezz7606
Copper(ii) sulfate pentahydrate, cuso4 ·5 h2o, (molar mass 250 g/mol) can be dehydrated by repeated heating in a crucible. which value is closest to the percentage mass of water lost from the total mass of salt in the crucible when the crucible undergoes repetitive heatings until a constant mass is reached? 25%13%36%26%

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
Copper(ii) sulfate pentahydrate, cuso4 ·5 h2o, (molar mass 250 g/mol) can be dehydrated by repeated...
Questions in other subjects:





Chemistry, 02.07.2019 22:50



Social Studies, 02.07.2019 22:50

Mathematics, 02.07.2019 23:00

Mathematics, 02.07.2019 23:00