
Alab scientist cools a liquid sample of water (2.6 kg) at 0.00°c to -192°c. the water turns to ice as this temperature change occurs. how much heat is released during this process? [for water, lf = 334 kj/kg and lv = 2257 kj/kg. the specific heat for ice is 2050 j/(kg·k)]. report your answer in kj. (round your answer to a whole number - no decimal places)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
Alab scientist cools a liquid sample of water (2.6 kg) at 0.00°c to -192°c. the water turns to ice a...
Questions in other subjects:

Chemistry, 23.11.2021 16:30



Mathematics, 23.11.2021 16:30


Mathematics, 23.11.2021 16:30

French, 23.11.2021 16:30

Social Studies, 23.11.2021 16:30

Arts, 23.11.2021 16:30

Mathematics, 23.11.2021 16:30


 ......(1)
......(1)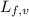 = latent heat of fusion or vaporization
= latent heat of fusion or vaporization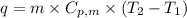 .......(1)
.......(1) = specific heat capacity of medium
= specific heat capacity of medium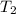 = final temperature
= final temperature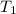 = initial temperature
= initial temperature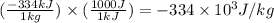

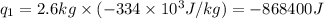
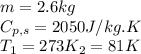
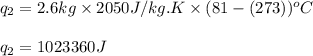
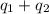
![[-868400+(-1023360)]J=-1891760J=-1891.76kJ\approx -1892kJ](/tpl/images/0420/8409/e23bd.png)


