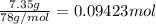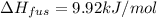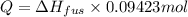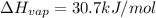Calculate the heat required to melt 7.35 g of benzene at its normal melting point. heat of fusion (benzene) = 9.92 kj/mol heat = kj
calculate the heat required to vaporize 7.35 g of benzene at its normal boiling point. heat of vaporization (benzene) = 30.7 kj/mol heat = kj

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, arnold2619
What is the force of attraction between the particles in a salt crystal
Answers: 2

You know the right answer?
Calculate the heat required to melt 7.35 g of benzene at its normal melting point. heat of fusion (b...
Questions in other subjects:


Spanish, 27.10.2019 06:43



Mathematics, 27.10.2019 06:43

Mathematics, 27.10.2019 06:43

Health, 27.10.2019 06:43



Physics, 27.10.2019 06:43