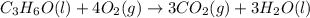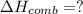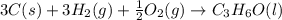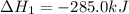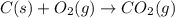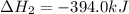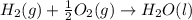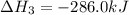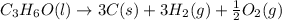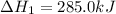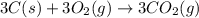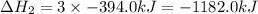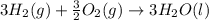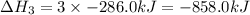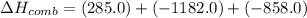Chemistry, 16.12.2019 17:31 gildedav001
What is the heat of reaction (δh°rxn) for the combustion of acetone (c3h6o) given the following thermochemical equations? 1. 3 c(s) + 3 h2(g) + ½ o2(g) → c3h6o(ℓ) δhf° = −285.0 kj 2. c(s) + o2(g) → co2(g) δhf° = −394.0 kj 3. h2(g) + ½ o2(g) → h2o(ℓ) δhf° = −286.0 kj

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 06:30, tdahna0403
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
What is the heat of reaction (δh°rxn) for the combustion of acetone (c3h6o) given the following ther...
Questions in other subjects:


Mathematics, 27.01.2021 22:30



Mathematics, 27.01.2021 22:30

Mathematics, 27.01.2021 22:30


Business, 27.01.2021 22:30

Geography, 27.01.2021 22:30

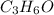 will be -1775 kJ
will be -1775 kJ