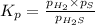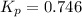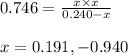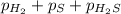Chemistry, 14.12.2019 06:31 nettaboo4664
At a certain temperature, the k p kp for the decomposition of h 2 s h2s is 0.746 0.746 . h 2 s ( g ) − ⇀ ↽ − h 2 ( g ) + s ( g ) h2s(g)↽−−⇀h2(g)+s(g) initially, only h 2 s h2s is present at a pressure of 0.240 0.240 bar in a closed container. what is the total pressure in the container at equilibrium?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 07:00, ultimatesaiyan
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
You know the right answer?
At a certain temperature, the k p kp for the decomposition of h 2 s h2s is 0.746 0.746 . h 2 s ( g )...
Questions in other subjects:



Computers and Technology, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01



Mathematics, 20.09.2020 14:01

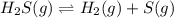
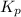 for above equation follows:
for above equation follows: