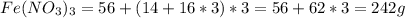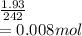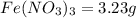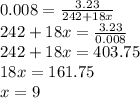Chemistry, 14.12.2019 06:31 zylandriawilliams
Astudent weighs out 3.23 g of fe(no3)3 • ? h2o and heats it. the remaining anhydrous salt has a mass of 1.93 g. calculate the empirical formula for the hydrate.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
Astudent weighs out 3.23 g of fe(no3)3 • ? h2o and heats it. the remaining anhydrous salt has a mass...
Questions in other subjects:

Mathematics, 09.07.2019 22:30

Mathematics, 09.07.2019 22:30


Mathematics, 09.07.2019 22:30

Mathematics, 09.07.2019 22:30

Mathematics, 09.07.2019 22:30

English, 09.07.2019 22:30



Mathematics, 09.07.2019 22:30

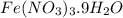
 '
'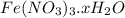
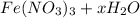
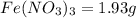
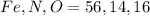 respectively.
respectively.