
Chemistry, 14.12.2019 06:31 braydenaddison738
Express the van der waals equation of state as a virial expansion in powers of 1/vm and obtain expressions for b and c in terms of the parameters a and b. the expansion you will need is (1 − x)−1 = 1 + x + x2 + … . measurements on argon gave b = −21.7 cm3 mol−1 and c = 1200 cm6 mol−2 for the virial coefficients at 273 k. what are the values of a and b in the corresponding van der waals equation of state?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, ashleyjaslin
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Express the van der waals equation of state as a virial expansion in powers of 1/vm and obtain expre...
Questions in other subjects:


History, 20.01.2021 17:00


English, 20.01.2021 17:00

Mathematics, 20.01.2021 17:00


Mathematics, 20.01.2021 17:00



English, 20.01.2021 17:00

![PV_{m} = RT[1 + (b-\frac{a}{RT})\frac{1}{V_{m} } + \frac{b^{2} }{V^{2} _{m} } + ...]](/tpl/images/0418/3833/bee7a.png)
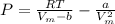
![P = RT[\frac{1}{V_{m}-b } - \frac{a}{RTV_{m} ^{2} }]](/tpl/images/0418/3833/a17f0.png)
![P = \frac{RT}{V_{m} } [\frac{1}{1-\frac{b}{V_{m} } } - \frac{a}{RTV_{m} }]](/tpl/images/0418/3833/173c2.png)
![PV_{m} = RT[(1-\frac{b}{V_{m} }) ^{-1} - \frac{a}{RTV_{m} }]](/tpl/images/0418/3833/0f37d.png)

![PV_{m} = RT[1+\frac{b}{V_{m} }+\frac{b^{2} }{V_{m} ^{2} } + ... -\frac{a}{RTV_{m} }]](/tpl/images/0418/3833/a2cf6.png)
![P = RT[\frac{1}{V_{m} }+ \frac{B}{V_{m} ^{2}}+\frac{C}{V_{m} ^{3} }+ ...]](/tpl/images/0418/3833/ad91d.png)
![PV_{m} = RT[1+ \frac{B}{V_{m} }+ \frac{C}{V_{m} ^{2} } + ...]](/tpl/images/0418/3833/3ea1d.png) equation (2)
equation (2) " alt="cm^{3}/mol" />" /> = 0.03464 L/mol
" alt="cm^{3}/mol" />" /> = 0.03464 L/mol

