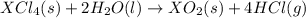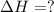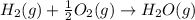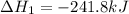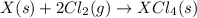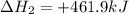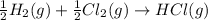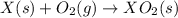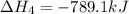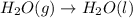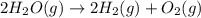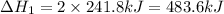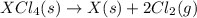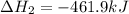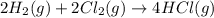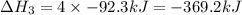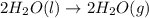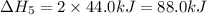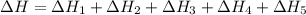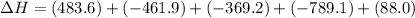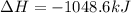Chemistry, 14.12.2019 05:31 ruddymorales1123
Given these reactions, where x represents a generic metal or metalloid 1 ) h 2 ( g ) + 1 2 o 2 ( g ) ⟶ h 2 o ( g ) δ h 1 = − 241.8 kj 2 ) x ( s ) + 2 cl 2 ( g ) ⟶ xcl 4 ( s ) δ h 2 = + 461.9 kj 3 ) 1 2 h 2 ( g ) + 1 2 cl 2 ( g ) ⟶ hcl ( g ) δ h 3 = − 92.3 kj 4 ) x ( s ) + o 2 ( g ) ⟶ xo 2 ( s ) δ h 4 = − 789.1 kj 5 ) h 2 o ( g ) ⟶ h 2 o ( l ) δ h 5 = − 44.0 kj what is the enthalpy, δ h , for this reaction? xcl 4 ( s ) + 2 h 2 o ( l ) ⟶ xo 2 ( s ) + 4 hcl ( g )

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
Given these reactions, where x represents a generic metal or metalloid 1 ) h 2 ( g ) + 1 2 o 2 ( g )...
Questions in other subjects:

Spanish, 20.10.2020 06:01

Biology, 20.10.2020 06:01


Biology, 20.10.2020 06:01