
Chemistry, 14.12.2019 03:31 KnMcdonaldk93906
Odetermine whether a shiny gold-colored rock is actually gold, a chemistry student decides to measure its heat capacity. she first weighs the rock and finds it has a mass of 4.7 g. she then finds that upon absorption of 57.2 j of heat, the temperature of the rock rises from 25 °c to 57 °c. find the specific heat capacity of the substance composing the rock and determine whether the value is consistent with the rock being pure gold.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

You know the right answer?
Odetermine whether a shiny gold-colored rock is actually gold, a chemistry student decides to measur...
Questions in other subjects:

Computers and Technology, 04.12.2020 17:00


Mathematics, 04.12.2020 17:00


Mathematics, 04.12.2020 17:00

Mathematics, 04.12.2020 17:00


English, 04.12.2020 17:00

History, 04.12.2020 17:00

Mathematics, 04.12.2020 17:00

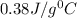 and the value is not consistent with the rock being pure gold.
and the value is not consistent with the rock being pure gold.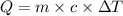
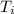 = 25.0°C
= 25.0°C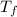 = 57.0°C
= 57.0°C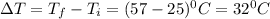
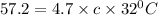
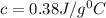
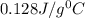 , thus the rock is not pure gold.
, thus the rock is not pure gold.

