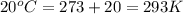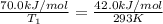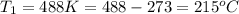Chemistry, 14.12.2019 01:31 ayoismeisjjjjuan
Apopular chemical demonstration is the "magic genie" procedure, in which hydrogen peroxide decomposes to water and oxygen gas with the aid of a catalyst. the activation energy of this (uncatalyzed) reaction is 70.0 kj/mol. when the catalyst is added, the activation energy (at 20.ºc) is 42.0 kj/mol. theoretically, to what temperature (ºc) would one have to heat the hydrogen peroxide solution so that the rate of the uncatalyzed reaction is equal to the rate of the catalyzed reaction at 20.ºc? assume the frequency factor a is constant, and assume the initial concentrations are the same. temperature = __ºc

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3


Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
Apopular chemical demonstration is the "magic genie" procedure, in which hydrogen peroxide decompose...
Questions in other subjects:


Chemistry, 01.10.2019 16:30


Mathematics, 01.10.2019 16:30


History, 01.10.2019 16:30


History, 01.10.2019 16:30

Social Studies, 01.10.2019 16:30

Biology, 01.10.2019 16:30

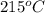
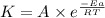
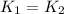
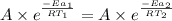
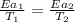 ..........(1)
..........(1)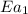 = activation energy for non-catalyzed reaction = 70.0 kJ/mol
= activation energy for non-catalyzed reaction = 70.0 kJ/mol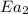 = activation energy for catalyzed reaction = 42.0 kJ/mol
= activation energy for catalyzed reaction = 42.0 kJ/mol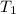 = temperature for non-catalyzed reaction = ?
= temperature for non-catalyzed reaction = ?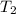 = temperature for catalyzed reaction =
= temperature for catalyzed reaction =