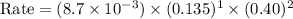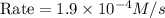Chemistry, 13.12.2019 23:31 albattatasraap5wymy
Consider the following reaction between mercury(ii) chloride and oxalate ion:
2hgcl2(aq)+c2o2−4(aq)→2cl−(aq)+2co2 (g)+hg2cl2(s)
the initial rate of this reaction was determined for several concentrations of hgcl2 and c2o2−4, and the following rate data were obtained for the rate of disappearance of c2o2−4:
experiment hgcl2(m) c2o2−4(m) rate (m/s)
1 0.164 0.15 3.2×10^−5
2 0.164 0.45 2.9×10^−4
3 0.082 0.45 1.4×10^−4
4 0.246 0.15 4.8×10^−5
what is the reaction rate when the concentration of hgcl2 is 0.135 m and that of c2o2−4 is 0.40 m , if the temperature is the same as that used to obtain the data shown?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, sillslola816oxb5h7
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 23.06.2019 04:10, NavyCo
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
Consider the following reaction between mercury(ii) chloride and oxalate ion:
2hgcl2(aq)+c2o2...
2hgcl2(aq)+c2o2...
Questions in other subjects:


Mathematics, 17.11.2020 06:30

English, 17.11.2020 06:30

History, 17.11.2020 06:30






Physics, 17.11.2020 06:30

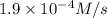
![\text{Rate}=k[HgCl_2]^a[C_2O_2^{4-}]^b](/tpl/images/0417/7719/2a902.png)
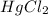
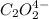
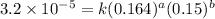 ....(1)
....(1)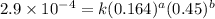 ....(2)
....(2)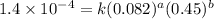 ....(3)
....(3)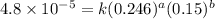 ....(4)
....(4)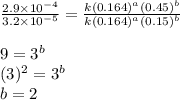
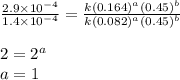
![\text{Rate}=k[HgCl_2]^1[C_2O_2^{4-}]^2](/tpl/images/0417/7719/2292e.png)
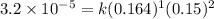
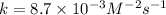
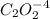 is 0.40 M.
is 0.40 M.