
For a first-order reaction, the half-life is constant. it depends only on the rate constant and not on the reactant concentration. it is expressed as: t1/2=0.693/k
for a second-order reaction, the half-life depends on the rate constant and the concentration of the reactant and so is expressed as: t1/2= 1/k[a]0
a. a certain first-order reaction (a--> products) has a rate constant of 3.30×10^-3 s^-1 at 45 degrees c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration?
b. a certain second-order reaction (b--> products) has a rate constant of 1.70×10^-3 m^-1*s^-1 at 27 degrees c and an initial half-life of 296 s. what is the concentration of the reactant b after one half-life?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 03:20, coollid876
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
For a first-order reaction, the half-life is constant. it depends only on the rate constant and not...
Questions in other subjects:

Social Studies, 07.10.2021 14:30



Mathematics, 07.10.2021 14:30



Mathematics, 07.10.2021 14:30

Mathematics, 07.10.2021 14:30


Law, 07.10.2021 14:30

![[A_t]=[A_0]e^{-kt}](/tpl/images/0417/6687/1ef89.png)
![[A_t]](/tpl/images/0417/6687/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0417/6687/9a686.png) is the initial concentration
is the initial concentration
![\frac {[A_t]}{[A_0]}](/tpl/images/0417/6687/0d33c.png) = 0.0625
= 0.0625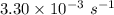
![\frac {[A_t]}{[A_0]}=e^{-k\times t}](/tpl/images/0417/6687/16cf4.png)
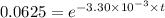
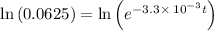
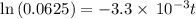
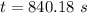
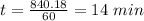
![t_{1/2}=\frac{1}{k[A_o]}](/tpl/images/0417/6687/4d220.png)
![[A_o]](/tpl/images/0417/6687/dc622.png) is the initial concentration = ?
is the initial concentration = ?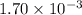 M⁻¹s⁻¹
M⁻¹s⁻¹
![296=\frac{1}{1.70\times 10^{-3}\times [A_o]}](/tpl/images/0417/6687/00cf0.png)
![296=\frac{1000}{1.7[A_o]}](/tpl/images/0417/6687/64d51.png)
![[A_o]=\frac{1250}{629}](/tpl/images/0417/6687/7cd99.png)
![[A_o]=1.99\ M](/tpl/images/0417/6687/59c0f.png)
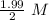 = 0.995 M
= 0.995 M

