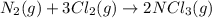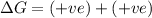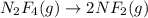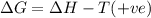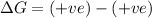Classify each of the following reactions as one of the four possible types:
1. spontan...

Chemistry, 13.12.2019 22:31 jose0765678755
Classify each of the following reactions as one of the four possible types:
1. spontaneous at all temperatures;
2. nonspontaneous at all temperatures;
3. spontaneous at low t; nonspontaneous at high t;
4. spontaneous at high t; nonspontaneous at low t.
(a) n2(g)+3f2(g)→2nf3(g); δh∘=−249kj; δs∘=−278j/k
(b) n2(g)+3cl2(g)→2ncl3(g); δh∘=460kj; δs∘=−275j/k
(c) n2f4(g)→2nf2(g); δh∘=85kj; δs∘=198j/k

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
Questions in other subjects:


Social Studies, 29.08.2019 10:00

History, 29.08.2019 10:00

Mathematics, 29.08.2019 10:00



English, 29.08.2019 10:00

Mathematics, 29.08.2019 10:00


Mathematics, 29.08.2019 10:00

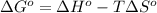
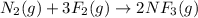
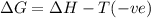
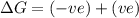
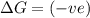 (at low Temperature)
(at low Temperature)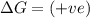 (at high Temperature)
(at high Temperature)