
Understanding the high-temperature formation and breakdown of the nitrogen oxides is essential for controlling the pollutants generated by car engines. the second-order reaction for the breakdown of nitric oxide to its elements has rate constants of 0.0796 l/mol-s at 737°c and 0.0815 l/mol-s at 947°c. what is the activation energy of this reaction? give your answer in scientific notation.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 23.06.2019 04:20, lelliott86
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1
You know the right answer?
Understanding the high-temperature formation and breakdown of the nitrogen oxides is essential for c...
Questions in other subjects:






Mathematics, 24.09.2021 01:50



Mathematics, 24.09.2021 01:50

Mathematics, 24.09.2021 01:50

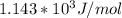
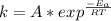
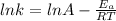
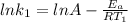 (1)
(1)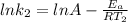 (2)
(2)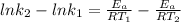 (3)
(3)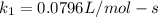 ;
; 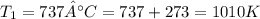
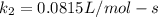 ;
; 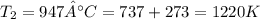
![ln 0.0815 - ln 0.0796 = E_{a}[\frac{1}{8.314*1010} - \frac{1}{8.314*1220}]](/tpl/images/0417/5925/574b6.png)


