
Chemistry, 13.12.2019 21:31 dornauriel
Two bulbs are connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxide at a pressure of 0.700 atm, 0.700 atm, and the small bulb, with a volume of 1.50 l, contains oxygen at a pressure of 2.50 atm. the temperature at the beginning and the end of the experiment is 22 ∘ c 22∘c . after the stopcock is opened, the gases mix and react. 2 no ( g ) + o 2 ( g ) → 2 no 2 ( g ) 2no(g)+o2(g)→2no2(g)
which gases are present at the end of the experiment?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, mimithurmond03
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2


Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
You know the right answer?
Two bulbs are connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxid...
Questions in other subjects:


Mathematics, 07.09.2021 05:30


Advanced Placement (AP), 07.09.2021 05:30




Mathematics, 07.09.2021 05:30


Mathematics, 07.09.2021 05:30

 and
and 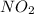
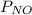 = 0
= 0

