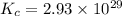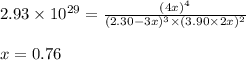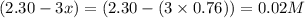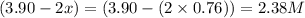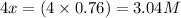Chemistry, 13.12.2019 18:31 zakarycrane9576
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, kc. 3 a ( g ) + 2 b ( g ) − ⇀ ↽ − 4 c ( g ) k c = 2.93 × 10 29 if, at this temperature, 2.30 mol of a and 3.90 mol of b are placed in a 1.00 l container, what are the concentrations of a, b, and c at equilibrium?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, danielahchf
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

You know the right answer?
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium consta...
Questions in other subjects:



English, 13.10.2020 14:01



Mathematics, 13.10.2020 14:01

Geography, 13.10.2020 14:01

Physics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

Biology, 13.10.2020 14:01

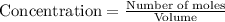
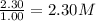
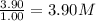
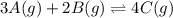
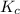 for above equation follows:
for above equation follows:![K_c=\frac{[C]^4}{[A]^3\times [B]^2}](/tpl/images/0417/2973/a92d9.png)