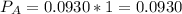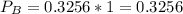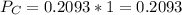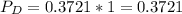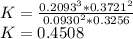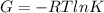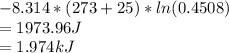Chemistry, 13.12.2019 01:31 Bryson2148
In the gas-phase reaction 2 a + b ⇌ 3 c + 2 d, it was found that, when 1.00 mol a, 2.00 mol b, and 1.00 mol d were mixed and allowed to come to equilibrium at 25 °c, the resulting mixture contained 0.90 mol c at a total pressure of 1.00 bar. calculate
(i) the mole fractions of each species at equilibrium,
(ii) k, and
(iii) δrimage.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
In the gas-phase reaction 2 a + b ⇌ 3 c + 2 d, it was found that, when 1.00 mol a, 2.00 mol b, and 1...
Questions in other subjects:



Mathematics, 07.03.2021 07:40

Mathematics, 07.03.2021 07:40



Mathematics, 07.03.2021 07:40

English, 07.03.2021 07:40

Mathematics, 07.03.2021 07:40

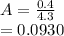
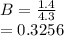
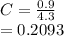
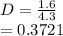
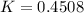
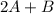 ⇄
⇄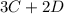
 be the number of moles dissociated per mole of
be the number of moles dissociated per mole of 
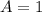
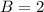
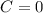
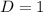
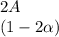 +
+ 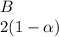 ⇄
⇄ 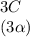 +
+ 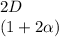
![C[tex] is 0.9Thus ,[tex]3\alpha=0.9\\\alpha=0.3[tex]The final number of moles of:[tex]A = 1-2\alpha=1-2*0.3=0.4mol[tex] [tex]B=2(1-\alpha)=2(1-0.3)=1.4mol[tex][tex]D=1+2\alpha=1+2*0.3=1.6mol[tex]Thus , total number of moles are : 0.4+1.4+0.9+1.6=4.3(i)The mole fractions are : [tex]A=\frac{0.4}{4.3} \\=0.0930](/tpl/images/0416/2107/4a2fd.png)
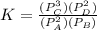
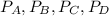 are the partial pressures of A,B,C,D respectively.
are the partial pressures of A,B,C,D respectively.