
Chemistry, 13.12.2019 01:31 terrysizemore666
The melting points for the second series transition elements increase from 1526˚c for yttrium to 2623˚c for molybdenum and then decrease again to 321˚c for cadmium. account for this trend in terms of band theory.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 23.06.2019 06:30, jamarstand
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
The melting points for the second series transition elements increase from 1526˚c for yttrium to 262...
Questions in other subjects:

Social Studies, 13.04.2021 02:00

Mathematics, 13.04.2021 02:00

Mathematics, 13.04.2021 02:00

Mathematics, 13.04.2021 02:00

Biology, 13.04.2021 02:00




Mathematics, 13.04.2021 02:00

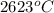 and after that the electrons goes into the antibonding and reaches minimum for cadmium when the anti boning orbital is completely filled. So, the melting point of the cadmium is tex]321^{o}C[/tex].
and after that the electrons goes into the antibonding and reaches minimum for cadmium when the anti boning orbital is completely filled. So, the melting point of the cadmium is tex]321^{o}C[/tex].

