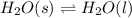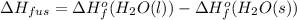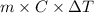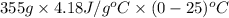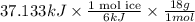Use the standard enthaplies of formation to calculate the standard change in enthaply for the melting of ice. (-291.8 kj/mol for h20 (s). use this value to calculate the mass of ice required to cool 355 ml of a beverage from the room temperature (25 degree celsius) to 0 degree celsius. assume that the specfic heat capacity and the density of the beverage are the same as those of water.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, WhiteWinterRose
What is the chemical formula of the following compound
Answers: 3

Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1


You know the right answer?
Use the standard enthaplies of formation to calculate the standard change in enthaply for the meltin...
Questions in other subjects:

Mathematics, 04.05.2020 23:33

History, 04.05.2020 23:33





Mathematics, 04.05.2020 23:33

Social Studies, 04.05.2020 23:33

History, 04.05.2020 23:33

Geography, 04.05.2020 23:33